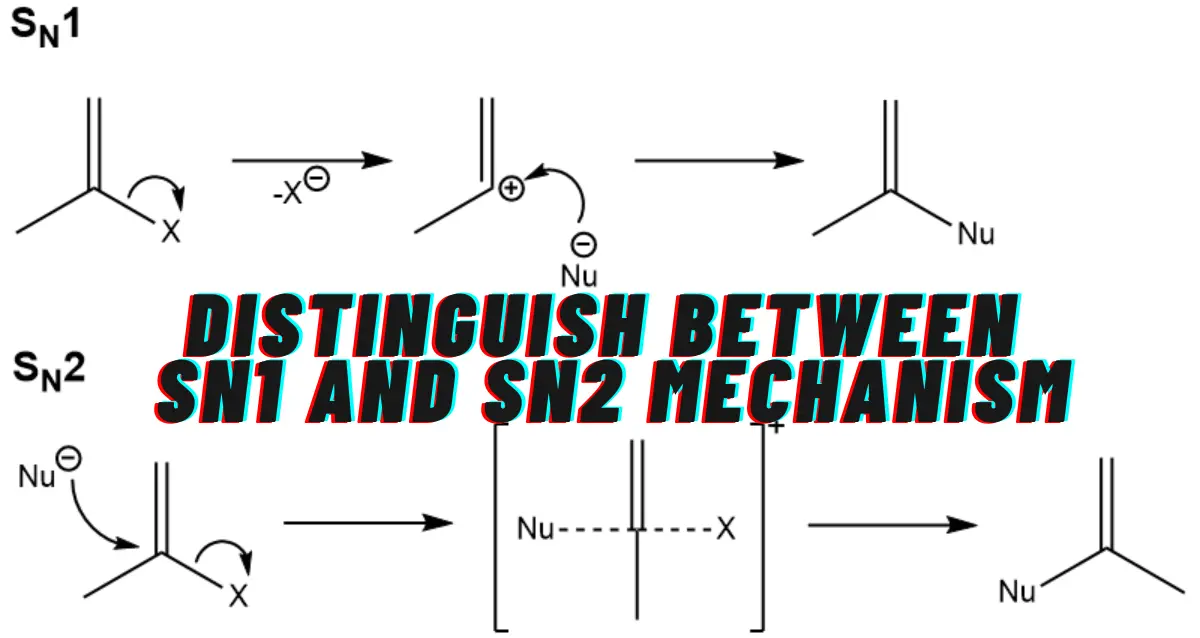
SN1 and SN2 are two different types of nucleophilic substitution reactions in organic chemistry. The main thing that distinguishes between SN1 and SN2 is their mechanism: SN1 reactions are unimolecular and proceed via a two-step mechanism, while SN2 reactions are bimolecular and proceed in one step. To understand the in-depth mechanism of SN1 and SN2 we need to first understand what is Nucleophile. Let’s jump directly into the details.
What is Nucleophile?
A nucleophile is a chemical species that has a pair of electrons and is capable of donating them to form a new chemical bond. The term nucleophile comes from the Greek words nucleus, which means the nucleus of an atom, and philos, which means loving. So, a nucleophile is essentially a nucleus-loving species, as it seeks to donate its electrons to an atom’s electron-deficient nucleus.
Nucleophiles play a crucial role in many chemical reactions, including the SN1 and SN2 reactions we’re discussing. They can be negatively charged ions (anions), neutral molecules, or even regions of a molecule that have a high electron density.
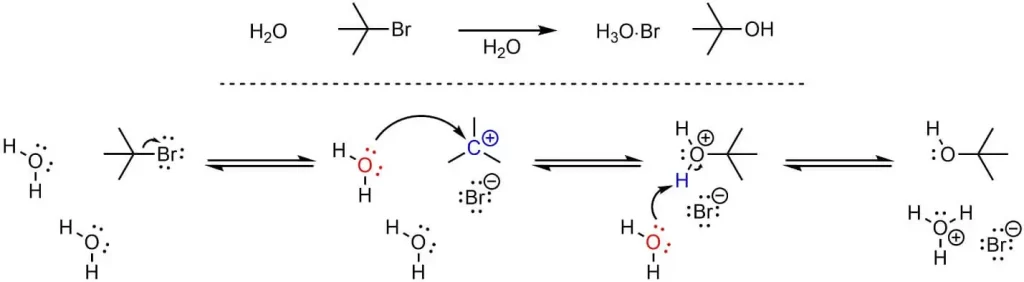
What is SN1 Reaction
SN1 stands for Substitution Nucleophilic Unimolecular, a type of substitution reaction where a nucleophile replaces a leaving group in a molecule. The unimolecular part of the name refers to the fact that the rate-determining step of this reaction involves only one molecule.
Example for SN1 Reaction
H2O, alcohols (ROH), CH3CN
Conditions favoring SN1 Reactions
Well, there are many reasons that can make SN1 reaction more likely to occur, few are below:
- SN1 reactions are favored by substrates that can easily form stable carbocations. For example, tertiary carbocations (those attached to three other carbon atoms) are more stable than secondary or primary carbocations.
- A good leaving group (one that can easily depart from the substrate) is necessary for an SN1 reaction. Halides like chloride (Cl-), bromide (Br-), and iodide (I-) are common leaving groups in SN1 reactions.
- Higher temperatures can increase the rate of SN1 reactions by providing the energy needed for the leaving group to depart.
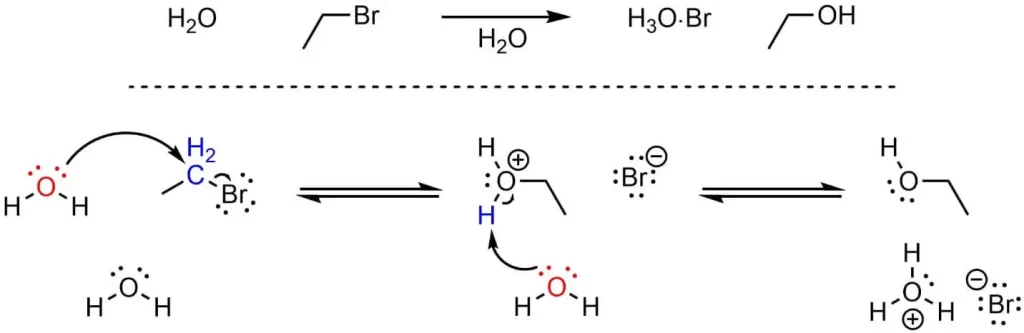
What is SN2 Reaction
SN2 stands for Substitution Nucleophilic Bimolecular, a type of substitution reaction that occurs in one step, with the nucleophile and substrate both involved in the rate-determining step. The nucleophile attacks the substrate, and the leaving group departs simultaneously.
The nucleophile attacks the substrate at the same time as the leaving group departs. This results in the inversion of the configuration at the carbon atom that was attached to the leaving group.
Example of SN2 Reaction
CH3CH2Br + OH- -> CH3CH2OH + Br-
Conditions favoring SN2 Reactions
Several factors can make an SN2 reaction more likely to occur:
- SN2 reactions are favored by substrates that are primary or methyl. This is because steric hindrance can prevent the nucleophile from attacking the substrate in SN2 reactions.
- Polar aprotic solvents, which cannot form hydrogen bonds, favor SN2 reactions. These solvents can stabilize the transition state. Examples of polar aprotic solvents include acetone and dimethyl sulfoxide.
- Strong nucleophiles favor SN2 reactions. A strong nucleophile is one that is eager to donate its electrons. Examples include hydroxide (OH-) and cyanide (CN-).
Comparison Between SN1 and SN2 Reactions
| Aspect | SN1 Reaction | SN2 Reaction |
| Definition | Unimolecular Nucleophilic Substitution | Bimolecular Nucleophilic Substitution |
| Rate-Determining Step | Formation of carbocation (slow step) | Simultaneous nucleophile attack and leaving group departure (rate-limiting step) |
| Reactivity | Tertiary > Secondary > Primary alkyl halides | Primary > Secondary > Tertiary alkyl halides |
| Stereochemistry | Racemization | Inversion of configuration (Stereospecific) |
| Nucleophile Influence | Weak nucleophiles preferred | Strong nucleophiles preferred |
| Solvent Influence | Polar protic solvents | Polar aprotic solvents |
| Reaction Mechanism | Stepwise mechanism (carbocation intermediate) | Concerted mechanism (no intermediate) |
| Rearrangement | Common due to carbocation stability | Rare, as it involves concerted reaction |
| Reaction Rate | Slower | Faster |
| Substrate Stereochemistry | No specific requirement | Requires the substrate to be chiral for retention or inversion of configuration |
| Reaction Conditions | Mild conditions | Requires good leaving group and strong nucleophile, often elevated temperature |
Watch this video for a better understanding of both reactions.
How SN1 and SN2 Reactions are Important
SN1 and SN2 reactions are fundamental to organic chemistry and have wide-ranging implications:
- These reactions are used in the synthesis of a variety of chemical compounds, which include many pharmaceutical drugs and commercially important chemicals.
- Many biological processes, such as DNA replication and protein synthesis, involve mechanisms similar to SN1 and SN2 reactions.
- Understanding these reactions can help in the development of more environmentally friendly chemical processes by optimizing reaction conditions to minimize waste and harmful byproducts.
If you want to improve your business then you may need to promote the education here is how you can do that.
FAQs
SN1 reactions like to happen in solvents that have a lot of positive and negative parts, like water or alcohol. They often involve breaking apart molecules by reacting with the solvent.
SN2 reactions prefer solvents that don’t have hydrogen atoms that can form bonds, like acetone or DMSO. These reactions usually involve the new molecule kicking out another atom or group of atoms.
It’s tough to tell SN1 and E1 apart because they both make carbocation steps and work better with weak bases or not-so-great nucleophiles.
Wrapping Up
SN1 and SN2 are two different types of nucleophilic substitution reactions in organic chemistry. They differ in their mechanisms, the conditions that favor them, and their practical applications. Understanding these reactions is crucial for anyone studying or working in the field of chemistry.